- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 5: e1. DOI 10.35630/2199-885X/2022/12/5.16
Background: The global prevalence of periodontal disease in adults is estimated up to 60-90%. Thus, development of new methods for diagnosis and treatment of periodontitis with their approbation on animal models is important. Aim: To create an experimental model of periodontitis in rats for the best possible simulation of clinical manifestations in humans according to the current understanding of the etiology and pathogenesis of this disease. Methods: The experiment was performed on 20 male Wistar rats (230±20 g). According to the proposed methodology, periodontitis was modeled by placing a metal ligature on the gingival margin of the lower incisors in a figure eight-shaped manner and fixing it to the alveolar bone thus destroying the ameloblastic epithelium. Periodontal inflammation was studied clinically and histologically. Microcirculation parameters were analyzed by Doppler ultrasound. Results: The use of the proposed periodontitis model was associated with inflammation, hyperperfusion causing an oxidative stress and periodontal destruction: Vas, Vakd and Vam significantly increased by 40.0% (p=0.007), 60.63% (p=0.005) and 83.33% (p=0.005), respectively. Also, there were significant decreases in the Gosling (PI) and Stewart (SD) indices: 63.82% (P=0.008) and 20.39% (p=0.005). Conclusion: The developed model is easily reproducible and similar to the clinical characteristics of periodontitis in humans, decrease the modeling time to 7 days thus simplifying the new developments in diagnosis and treatment of periodontitis.
Keywords: chronic stress; experimental periodontitis; microcirculation; Wistar rats
Periodontitis is one of the most persistent problems of dentistry. The global prevalence of periodontal disease in adults reaches 60-90% [1]. The current issues related to the etiology, pathogenesis and clinical characteristics of chronic periodontitis are associated with the prevalence of periodontitis in the elderly and younger working-age people, as well as the low efficacy of available treatment methods [2, 3]. Thus, development of new methods of periodontitis treatment and diagnosis in animal models is important.
Currently there are a number of experimental models of periodontitis in rodents, rabbits, pigs, dogs and primates, which are widely used for assessment of the efficacy of new treatment methods; each model has its advantages and disadvantages [4, 5]. A common experimental model of periodontitis uses a diet high in sucrose and casein. Periodontitis developed without mechanical intervention in the oral cavity and in the absence of any noticeable metabolic disorders [6]. There is also a periodontitis model that involves placing a 3/0 size silk ligature on the first left molar teeth of the lower jaw of rats, with the ligature removed in 14 days [7]. Experimental periodontitis may also be modeled by injection of lipopolysaccharide (LPS) of Porphyromonas gingivalis (Pg) in rats [8].
The animal models yielded a wide range of important results reflecting the pathogenic characteristics of periodontitis [9, 10]; new methods of prevention and treatment of inflammatory periodontal disease were developed. The question remains, however, whether the results can be extrapolated to humans, as the development of periodontitis in humans depends on the structural characteristics, reactivity and resistance to microorganisms [11, 12].
Aim: To create an experimental periodontitis model for the best possible simulation of the pathomorphological and clinical manifestations in humans according to the current understanding of the etiology and pathogenesis of this disease.
The experiment was performed on 3-month-old 20 male Wistar rats with a body weight of 230±20 g, obtained from the Rappolovo laboratory animal nursery (Russia).
The animals were kept in cages (10 animals in each) with free access to food and water and fed once a day (from 9 to 10 a.m.). The rats were housed in a room with controlled temperature (21±1°С) and humidity (50-55%) under natural light conditions.
This experimental study in animals was approved by the Ethic Committee (Meeting Minutes No. 6 of January 24, 2019). The study was conducted according to the ethical principles established by the Declaration of Helsinki.
Under the proposed methodology, periodontitis was modeled by placing a metal ligature (aluminum ligature wire for splinting teeth with jaw fractures, diameter 0.5 mm) on the gingival margin of lower incisors in a figure eight-shaped manner, using Zoletil 100 (France) (01 ml/100g intraperitoneally) for analgesia. The ligature was fixed to the alveolar process by a silk suture thus disrupting the integrity of the ameloblastic epithelium (hydrostatic pad) at the vestibular side where it has the lowest thickness, in order to create a site of entry for microorganisms. From Day 2 to Day 7 of the experiment, each animal was placed in a cage with a surface area of 0.0008 m2 for 6 hours daily to induce a chronic immobilization stress (IS) as one of the risk factors of periodontitis. Throughout the experiment the animals were receiving a high-carbohydrate diet consisting of wheat porridge with added milk (30%), starch (20%) and sugar (15%), which was prepared daily [13].
Clinical signs were assessed on Days 1 and 7 (D1 – intact periodontal tissue), D7 – experimental periodontitis). To assess the grade of periodontal inflammation, a severity of periodontitis (SP) index was developed. The rating scale was the following: 0 – intact periodontal tissue (pale pink gingiva without bleeding); 1 – pale pink gingiva with bleeding on probing, no gingival pockets; 2 – loose gingival tissue with hyperemia and edema, bleeding on probing, gingival pockets up to 1.5 mm deep, mobility grade I; 3 – gingival hyperemia and edema with severe bleeding on probing, gingival pockets up to 3 mm deep, mobility grade II.
A small sample of gingival tissue (including free gingiva, gingival papilla) was taken for histopathological study on D1 and D7 and fixed in 10% formalin solution. Fixed tissues were washed under running tap water, dehydrated with acetone, cleaned in benzene and embedded in paraffin (melting point 60-62 C). Paraffin slides with a thickness of 4-5 μm were stained with hematoxylin and eosin (H&E). They were examined using a ZEISS Axio Lab.A1 polarization microscope with a digital camera (Germany). The following histological parameters were assessed: 1) inflammation severity; 2) connective tissue structure.
Doppler ultrasound was used to detect microcirculation disorders (Angiodin-PK, Russia, 16 MHz probe). Each rat was fixed on a wooden board in the supine position with the upper and lower jaws fixed open. A transitional gingival fold area close to the lower incisors with no large blood vessels was chosen for assessment of the fluid exchange in the gum tissue: systolic (Vas), diastolic (Vakd) and mean (Vam) blood flow velocity; PI – pulsation index (Gosling index); RI – peripheral resistance index (Purcell index); SD – systolic-diastolic index (Stewart index).
The data were analyzed using GraphPad Prism 7 (GraphPad Company, USA). Median (25–75‰) values were given due to a small number of variants in the sample (Wilcoxon’s test). Results were considered statistically significant with P<0.05.
On D1 the gingiva was pale pink and smooth (Fig. 1А). SP index = 0. Histological examination in healthy animals on Day 1 of the experiment before ligature placement revealed stratified squamous keratinized epithelium in the gingiva. The mucosal lamina propria appeared as relatively thin and organized collagen fibers with inactive fibrocytes between them. A small number of arterioles, venules and capillary vessels were observed (Fig. 1B).
On D7, mineralized dental plaque was observed with gingival pockets up to 2 mm deep and grade I and II tooth mobility (Fig. 1C). SP index was 2.65±0.11. Histological examination of the gingival tissues showed acute inflammation: edema and collagen fiber destruction with formation of highly vascularized granulation tissue with large numbers of histiocytes, lymphocytes and plasma cells in all animals. The vessels were dilated throughout, with several foci of perivascular edema and congestion in some vessels (Fig. 1D).
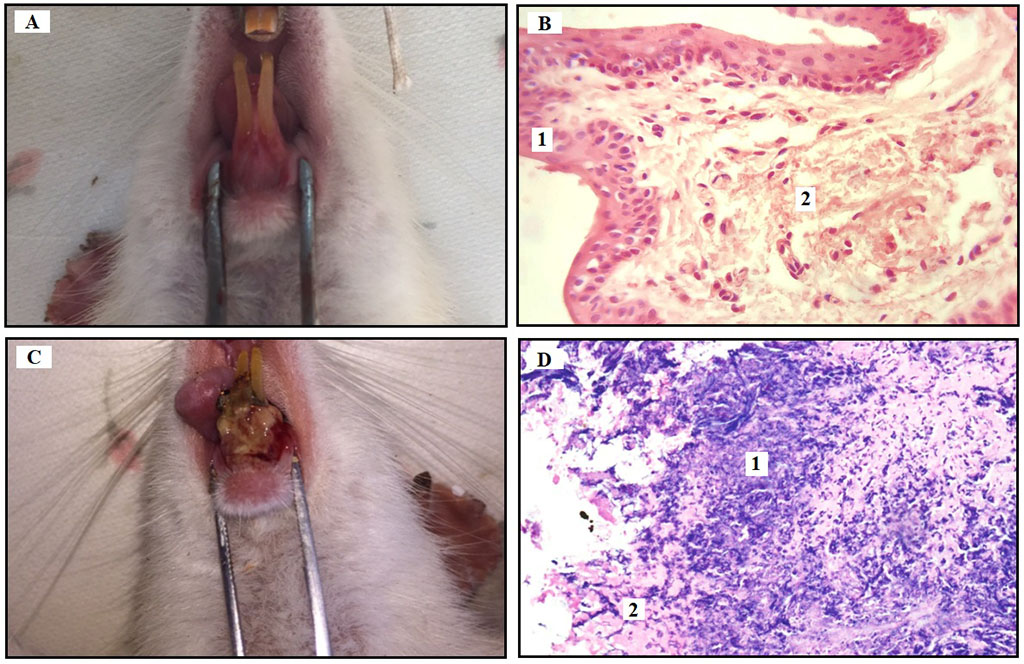
Fig. 1. Clinical and histological findings for each experimental day (D1, D7).
Note: (A) Clinical aspect of healthy gingiva on D1; (B) Histological presentation of the gingival stratified squamous keratinized epithelium (1) and lamina propria consisting of connective tissue (2) (H&E, bar=40 nm); (C) Clinical aspect of periodontitis on D7: gingival cyanosis, ulcer covered by debris, spontaneous bleeding, gingival retraction, destruction of alveolar bone and periodontal ligament; (D) Swelling and destruction of collagen fibers (1), the formation of highly vascularized granulation tissue (2) (H&E, bar=40 nm).
In animals with experimental periodontitis, the Vas, Vakd and Vam were significantly higher on D7 compared with the control (D1): by 40.0% (p=0.007), 60.63% (p=0.005) and 83.33% (p=0.005), respectively.
Analyzing the elasticity of the blood vessels, we revealed significant decreases in the Gosling (PI) and Stewart (SD) indices in experimental periodontitis: 63.82% (P=0.008) and 20.39% (p=0.005), respectively. The peripheral resistance index (RI) was also lower on D7, but there were no significant differences with D1.
Thus, local inflammation was associated with increases in systolic (Vas) and diastolic (Vakd) blood flow velocities, statistically significant hyperperfusion (increased Vam), and a decrease in permeability and peripheral resistance of blood vessels contributing to oxidative stress and periodontal alteration (Fig. 2).
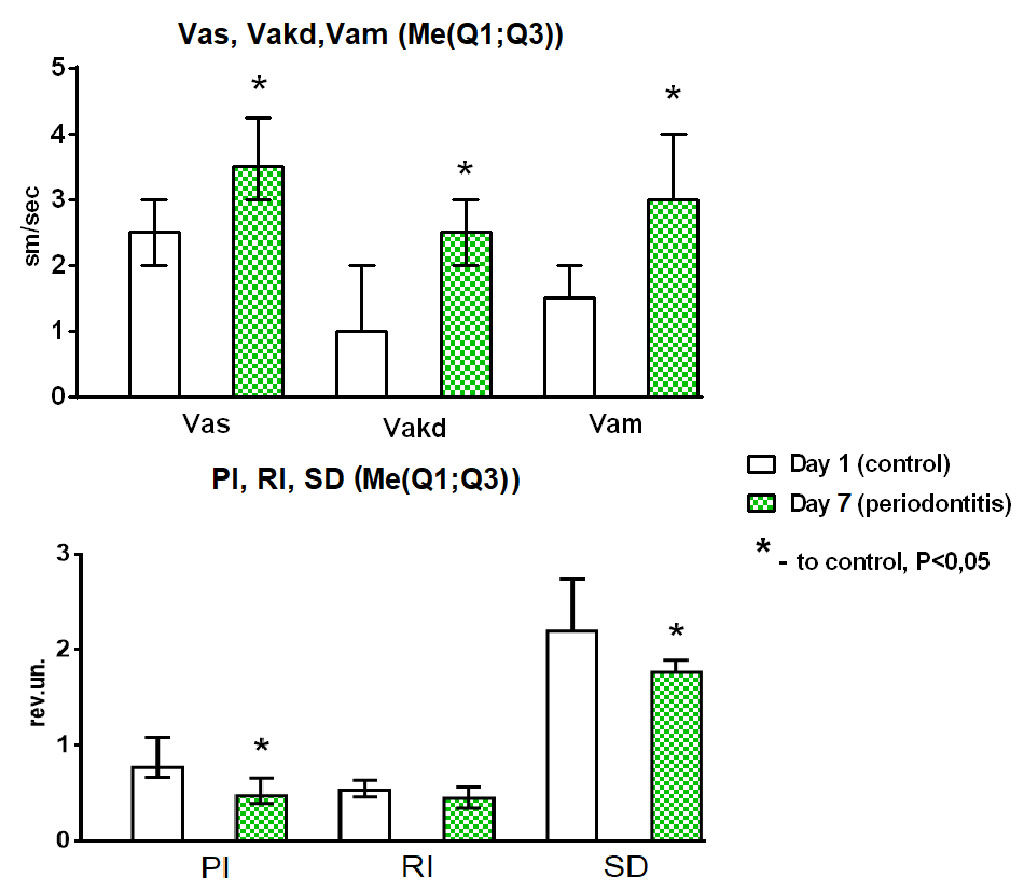
Fig. 2. Changes over time in microcirculation parameters.
Note: systolic flow velocity (Vas, cm/sec), diastolic flow velocity (Vakd, cm/sec), medium flow velocity (Vam, cm/sec), pulsatility index (PI), resistance index (RI), Stuart index (SD) in the transitional gingival fold attachment area. Control (microcirculation parameters on D1 – healthy periodontium); D7 – microcirculation disorders on D7 – experimental periodontitis; *Statistically significant difference compared to control, P<0.05 (Box Plot of multiple variables, n=20).
Hyperperfusion leads to oxidative stress increasing lipid peroxides contents. Polymorphonuclear leukocytes (PMNLs) are the first line of defense against periodontal pathogens. PMNLs as a part of the oxygen-dependent mechanism produce a large number of reactive oxygen species (ROS) during phagocytosis of periodontal pathogens [14, 15]. ROS are highly toxic substances produced in normal physiological conditions and then removed by antioxidant systems to prevent tissue damage. Connective tissue cells and intercellular matrix are deficient in antioxidant defense enzymes, lipids and water-soluble antioxidants, and thus are not resistant to excessive lipid peroxidation products. ROS thus inhibit collagen synthesis [14]. Chronic stress used as a general factor in the current model stimulates the discharge of a large number of superoxide anions into the intercellular matrix thus promoting a higher degree of disorganization of periodontal connective tissue structures. Physiological reactions to chronic stress modify the immune system through nervous (autonomous nervous system) and endocrine (hypothalamic-pituitary-adrenal axis) systems [16, 17].
The results of the study show that the ligature induced periodontitis with damage to the ameloblastic epithelium, which prevented periodontal repair and promoted accelerated inflammation with clear clinical signs such as edema, bleeding, buildup of heavy soft dental plaque, development of gingival pockets up to 2 mm deep and lower mobility of the incisor teeth. Periodontal inflammation in lower incisor teeth was found in 100% of the animals. Histological examination of the gingiva on Day 7 showed signs of inflammation thus confirming the achievement of the study aim to induce experimental periodontitis.
Ligature (silk or metal)- and bacteria-induced experimental periodontitis models are most commonly used [18]. Induction of ligature-induced periodontitis in rats has several advantages, including the low costs of small animals and the availability of germ-free rats. Ligature placement itself does not induce significant inflammation and alveolar bone loss, but germ-free rat and mouse models have demonstrated that the inflammation and bone loss depend on the accumulation of bacteria on the ligature (the interaction between oral microorganisms and host responses). The ligature-induced rat periodontitis model has the benefit of demonstrating the host response, because rats display fewer individual differences than humans [19, 20]. Despite this, ligature-induced periodontitis has several disadvantages: technical difficulty in placing the ligature around the molars due to the small size of the oral cavity and teeth (ligature placement requires a very high level of technical skill). Also, the ligature itself will rarely induce marked periodontal inflammation, the severity of which depends on the body’s reactivity and external factors [21].
Also, oral administration of Porphyromonas gingivalis and local injection of lipopolysaccharide are also commonly used methods for inducing periodontitis. The periodontal destruction in humans is due to microorganisms, therefore, host immune response against bacteria plays a very important role in the progression of alveolar bone loss. These two methods take a long time (over 4 months) to produce a significant loss of periodontal tissue [18].
Periodontitis is a multifactorial disease. There are two mechanisms of periodontal destruction [22, 23]. The first includes toxins, lipopolysaccharides and microbial enzymes. The second involves activation of the immune system producing neutrophils, macrophages, lymphocytes and high concentrations of cytokines and other inflammatory mediators, and osteoclast activation. Besides, risk factors such as decreased reactivity, stress and dietary habits (general factors) aggravate periodontal inflammation [24].
Wistar rats were chosen as the experimental model for this study mostly because the structure of the gingival margin, according to literature data, is similar to that in humans [25]. Horizontal bone loss was observed in rats infected with Porphyromonas gingivalis, Aggregatibacter actinomycetem comitans, which may be used in experimental model development and research of periodontal tissue regeneration using new treatment methods [22, 23]. Second, rats are most commonly used for experimental modeling as they are resistant to stress. The structure of their periodontal tissue, however, has a number of characteristics providing for natural resistance to periodontal disease, which should be kept in mind during experiments; these include cartilage joint of alveolar ridges, bent root shape, hydrostatic pad at the root curvature area covered with ameloblastic epithelium and facilitating periodontal regeneration, and differences in oral microbiota [24, 26].
Similarly to human periodontitis, Porphyromonas gingivalis is found by microbiological tests in rat gingival pockets. Pg virulence components, such as lipopolysaccharide, lipoproteins, gingipains and fimbriae, play an important role in the induction of immune reactions, including cytokine production and activation of inflammatory signaling pathways. Inflammatory stimuli, such as PAMPs (pathogen-associated molecular patterns) and DAMPs (danger-associated molecular patterns), are recognized by TLRs (toll-like receptors), which in turn induce NF-κB (receptor activator of nuclear factor kappa-B)-mediated upregulation of NLRP3 (NOD-like receptor pyrin domain-containing protein 3), pro-caspase-1, pro-IL-1β, and pro-IL-18. NLRP3 would associate with ASC (caspase-1 recruitment domain) specks and pro-caspase-1 to form multi-protein complexes (NLRP3 inflammasome). NLRP3 inflammasome activation leads to the maturation and secretion of IL-1β and IL-18. Bacterial infection in periodontium leads to the activation of the NLRP3 inflammasome in osteoclast precursor cells, which promotes osteoclast differentiation and alveolar bone resorption (Fig. 3). A ligature increases IL-1β production in periodontal tissue due to more pronounced absorption of microorganisms around it, which in turn induces RANKL expression and activity thus starting the process of osteoclastogenesis and bone loss. Immobilization stress used in the model leads to the release of glucocorticoids. They promote LPS-induced NF-kB activation, increase IL-1β, IL-6 and IL-8 secretion, nuclear factor of activated T-cells (NFATc1), and inhibit osteoprotegerin (OPG) (Fig. 3) [27].
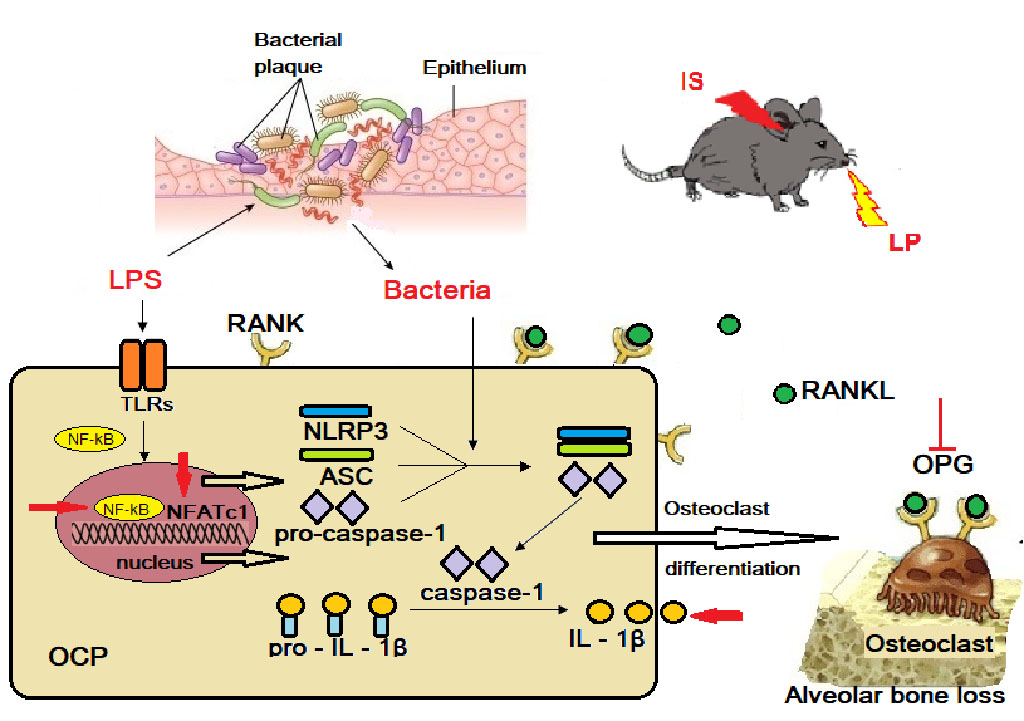
Fig. 3. Schematic diagram of NLRP3 regulating alveolar bone loss in ligature-induced periodontitis.
Note: the red arrows indicates the effects of chronic stress on alveolar bone resorption; IS – immobilization stress; LP – ligature placement; OCP – osteoclast precursor; OPG – osteoprotegerin; LPS – lipopolysaccharide; ASC – caspase-1 recruitment domain; TLRs – toll-like receptors; RANKL – receptor activator of nuclear factor kappa-B ligand; NF-κB – receptor activator of nuclear factor kappa-B; NFATc1 – nuclear factor of activated T-cells.
The carbohydrate-rich food causes changes in metabolism, to a greater extent lipid metabolism, with abnormal accumulation of triglycerides, mitochondrial dysfunction, inflammation and oxidative stress. In this study, the animals were on a high-carbohydrate diet (wheat porridge with starch (20%), milk (30%), and sugar (15%)) [13], which aggravated the oxidative stress in animals, and also contributed to the accumulation of plaque in the ligature area, serving as a good nutrient medium for microorganisms. OS is closely related to cardiovascular diseases; in particular, it plays one of the key roles in the pathogenesis of endothelium-dependent vasodilation in cardiovascular diseases. This violation of microvascular blood flow is mediated by endothelial dysfunction [28]. It follows that a high-carbohydrate diet aggravates the severity of the inflammation due to both local and systemic effects.
The proposed model has the following advantages: a lower incisor periodontitis model is convenient for daily observation; the best possible clinical and pathomorphological simulation of the human pathogenesis is achieved within 7 days due to several etiologic factors. Local factor: the metal ligature fixed to the alveolar process by a silk suture thus disrupting the integrity of the ameloblastic epithelium (hydrostatic pad) at the vestibular side where it has the lowest thickness, excluding periodontal regeneration by its cells and maintaining the alteration. General factors: the high-carbohydrate diet creating a suitable environment for periodontal pathogenic microbiota and the immobilization stress activating a systemic inflammatory response.
The limitation of this model is that the root area of lower incisors, as opposed to molar teeth, has ameloblastic epithelium that restores periodontal tissue in rats, but we fixed the metal ligature to the alveolar process in order to disrupt the integrity of this epithelium. The limitation of the rat as an experimental periodontitis model is epithelial keratinization, which may present a protective mechanism against microbial colonization; the presence of a ligature, however, promotes increased adhesion of periodontal pathogens.
The developed model is easily reproducible; it decreases the modeling time to 7 days thus accelerating trials of new medications and periodontitis treatment methods. Blood flow velocity changes in gingival tissue are an important early diagnostic criterion of periodontal destruction, which may be of great value in the development of new early diagnostic and treatment methods in experimental animals, when the disease is more susceptible to non-operative treatment.
In summary, our findings in rats provide a consistent experimental model with inflammatory cell infiltration and alveolar bone resorption to evaluate the pathogenesis of periodontitis and assess the action of novel drugs or treatments prior to clinical trials in periodontal research.